
What is Soap?
Published by Anne Altor on Mar 3rd 2019
Have you ever wondered what soap is, or been curious about the science behind the bubbles?
The magical kettle of soapy stew holds lessons in science for me and you!

You don't have to be a chemistry major to be interested in or understand the basic science of soap. My goal in this post is to make this subject a little more digestible. If you'd like to learn more about One Earth soap, you can check it out here.
To understand soap, we first need a handle on fats and oils!
Fats and oils are composed of fatty acids and glycerides. What are those?
FATTY ACIDS:
An acid is any molecule that can donate a hydrogen atom (H+). A "fatty" acid has a carboxyl group (COOH) attached to a hydrocarbon chain (a chain of carbon and hydrogen atoms). The carboxyl group can release its H+, which makes the molecule an acid. Stearic acid (C17H35COOH) is a common fatty acid in many oils and fats. Here's a cartoon of stearic acid (black balls are carbon atoms, white balls are hydrogen atoms, red balls are oxygen atoms):


Its simple line structure is:


The particular fatty acids in soap determine the qualities of the soap, such as cleansing, lather, hardness, conditioning. Each oil or fat has a particular mixture of fatty acids. The major fatty acids in soapmaking oils are:
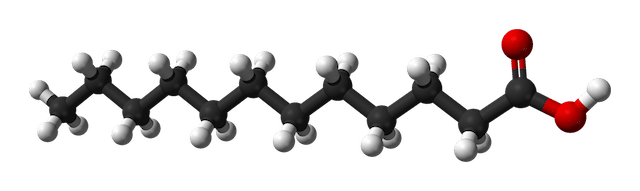
Lauric acid, C12H24O2
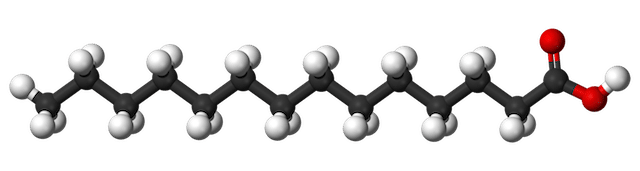
Myristic acid, C14H28O2
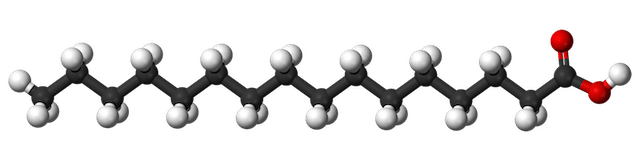
Palmitic acid, C16H32O2
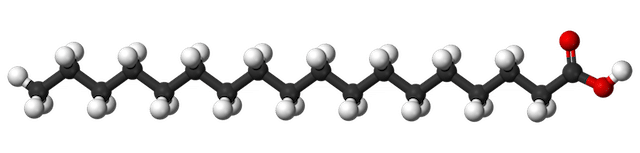
Stearic acid, C18H36O2
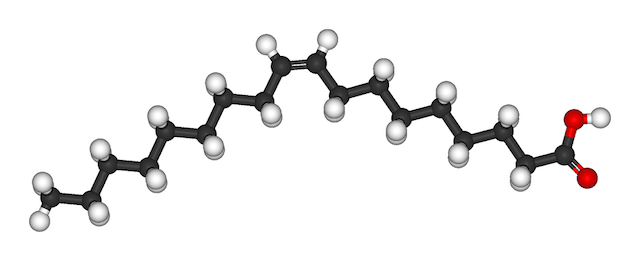
Oleic acid, C18H34O2
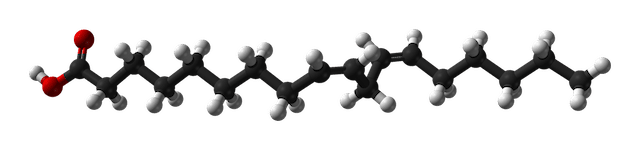
Linoleic acid, C18H32O2
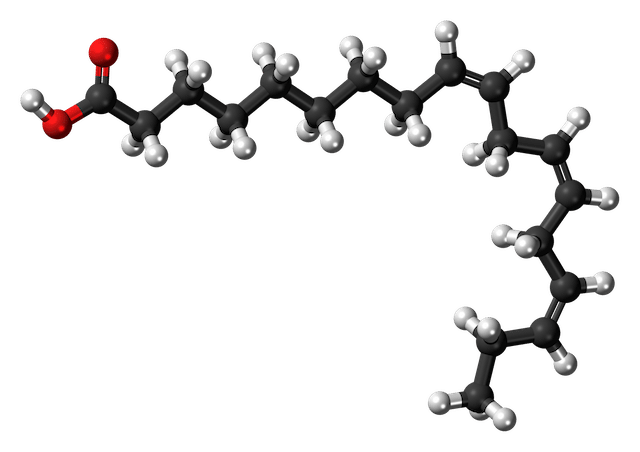
Alpha Linolenic acid, C18H30O2
(Images from Wikimedia Commons)
As you can see, the fatty acids have different "chain lengths" and shapes because they have different numbers and configurations of carbon atoms. The straight-chain fatty acids are dominant in saturated fats and butters, where every carbon has single bonds with other carbons and hydrogens (except for bonds with oxygen at the end of the chain). The bent fatty acids have double bonds between one or more carbon atoms and so are unsaturated. The molecules have a kinked configuration of C and H atoms around the double bonds, compared to the even zig-zag pattern where each C is attached to 2 other carbons and 2 hydrogen atoms. Unsaturated fatty acids are dominant in oils that are liquid at room temperature (around 70°F or 21°C).
But why does the shape of the fatty acid affect whether a fat or oil is solid or liquid at room temperature?
Straight-chain fatty acids can pack closely together, which leads to increased strength of intermolecular attraction between the fatty acid molecules, thus raising the melting temperature. In contrast, unsaturated fatty acids can't pack closely together, so the intermolecular attraction between fatty acid molecules is weaker and they melt at lower temperatures.
GLYCERINE:
Glycerine (C3H8O3, below) is a molecule with 3 carbon atoms attached to 5 hydrogen atoms and 3 OH groups.


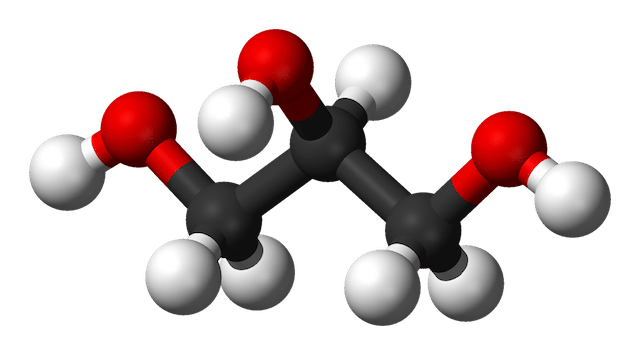
Glycerine, C3H8O3. Images from Wikimedia Commons.
The OH groups of glycerin react with the COOH groups of fatty acids, releasing water and forming glycerides. When 3 fatty acids react with one glycerine molecule, the result is a triglyceride.
Below is an example of a triglyceride. The left part of the molecule shows the glycerine-based backbone; the leftmost O atoms (attached to C atoms) are at the location where OH groups of the glycerine molecule reacted with COOH groups of the fatty acids, releasing H2O and joining the molecules:
Fats are made of triglycerides.
OK! But what is SOAP??
When oils and fats are mixed with a strong base (sodium or potassium hydroxide), soap is made! A base is a molecule that can release an OH– group. Lye is a solution of sodium or potassium hydroxide (NaOH or KOH) dissolved in water. When lye made with sodium hydroxide reacts with triglycerides, it breaks the bonds between the glyceride part of the molecule and the fatty acid parts. The glyceride gets its Hydrogens back to become glycerine again, and the fatty acids acquire sodium (Na+), becoming "salts."
For example, when stearic acid reacts with lye, it loses a Hydrogen (from the OH on the end of the molecule) and attracts a sodium ion (Na+).


Stearic Acid Sodium Salt Structural Formula, Wikimedia Commons
So SOAP is a mixture of salts of fatty acids and glycerine, and it has a high (basic) pH because lye is a strong base that releases OH– molecules.
